Stone Capital Group partners with LumiraDx to offer comprehensive COVID-19 testing solutions for mass screening settings
Results are encouraging
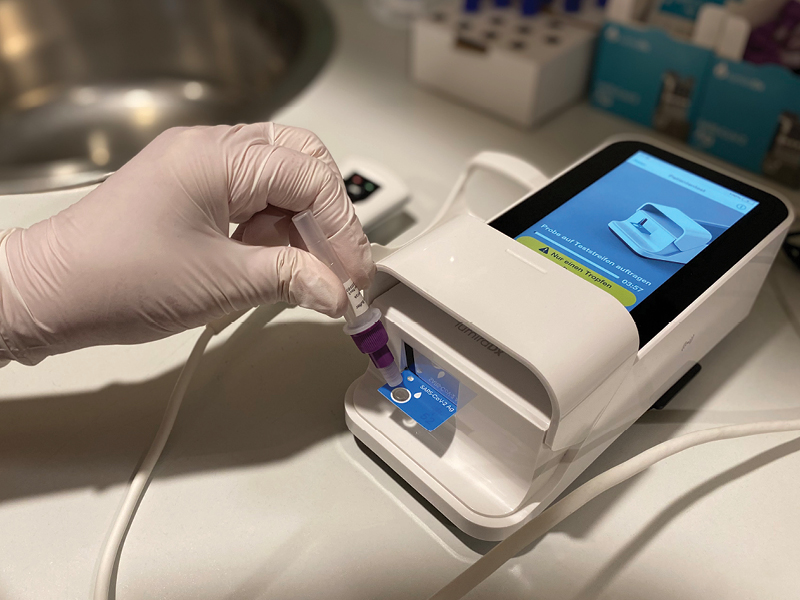
May 24th, 2021: Stone Capital Group, a company comprised of high-complexity clinical reference laboratories, distribution, and mobile laboratory business unit (#Stone Direct Diagnostics) announced today that the Company has entered a strategic Services and Co-Marketing partnership with LumiraDx, a next-generation point of care (POC) diagnostics testing company. This partnership will focus on expanding Stone’s ability to provide a complete portfolio of fast, accurate testing from the clinical laboratory to non-laboratory settings including the cutting-edge molecular lab LumiraDx SARS-CoV-2 RNA STAR Complete reagents and the high sensitivity point of care (POC) LumiraDx SARS-CoV-2 Antigen Test.
The partnership allows Stone to provide LumiraDx’s unique COVID-19 testing solutions across a number of settings where where diagnostic testing and screening of COVID-19 is critical to ensuring the health and safety of groups. This includes Stone’s current slate of customers including movie and production studios, government agencies, professional sports organizations, schools, universities, large employer groups and cultural and entertainment events including last week’s Food Network “South Beach Wine & Food Festival (SoBe)” in Miami Florida, as well as the city’s upcoming “Air & Sea Show & Music Explosion”.
Stone Capital Group’s CEO and Founder Christopher Ridgeway, explained, “Stone is extremely excited to partner with LumiraDx to enable us to offer best-in-class COVID-19 testing platform, that is both fast and extremely accurate. We are committed to providing our clients with flexible and full-service clinical support. We see this partnership as an important strategy in continuing our efforts to deliver boutique high complexity testing services along with best in class point of care platforms to our members within our managed care networks of more than 220 million lives.”
LumiraDx’s RNA STAR complete offers high sensitivity, high throughput molecular PCR testing on wheels. With a direct amplification method that does not require any sample preparation equipment and reagents, RNA STAR Complete enables a single mobile laboratory with 3 instruments to test 1000 individuals per hour. This is complemented with high sensitivity antigen testing with the LumiraDx’s SARS-CoV-2 Antigen test with results in 12 minutes for immediate results.
LumiraDx’s President, North American Commercial Operations Peter Scheu commented, “Partnering with Stone is an incredible opportunity to bring our COVID-19 testing solutions to diagnostic as well as mass screening settings. Providing rapid and accurate test results instills confidence to groups, whether they are event attendees, employees, or students, by knowing their health is of utmost concern and the environment is secure. This type of comprehensive testing is critical as we work to safely reopen our communities and return to normal.”
About Stone Capital Group:
Founded in 2016 Stone Capital Group focuses on simplifying testing, improving the efficiency of molecular diagnostics, reducing consumer and corporate cost, and increasing overall access to innovative diagnostics and the quality of patient care. Stone’s high-complexity laboratories use PCR-based molecular testing to detect infectious diseases and other DNA/RNA based testing, while continually enhancing its offerings in complementary diagnostic services including chemistry, hematology, microbiology, pharmacogenetic, prescription drug monitoring and point of care testing.
About LumiraDx
Founded in 2014, LumiraDx develops, manufactures, and commercializes an innovative point of care diagnostic Platform. The LumiraDx Platform is designed to deliver lab comparable diagnostic results at the point of care in minutes. It is designed to be affordable and accessible for healthcare providers globally, and to strengthen community-based healthcare.
The LumiraDx SARS-CoV-2 RNA STAR Complete and the LumiraDx SARS-CoV-2 Antigen test have not been cleared or approved by the FDA. The tests have been authorized by FDA under an EUA only for the detection of SARS-CoV-2 nucleic acid and SARS-CoV-2 nucleocapsid protein respectively. The test has not been authorized for use to detect any other viruses or pathogens.
The LumiraDx SARS-CoV-2 Antigen test is intended for the qualitative detection of nucleocapsid protein antigen to SARS-CoV-2 directly from nasal swab specimens collected from individuals suspected of COVID-19 by their healthcare provider within the first 12 days of symptom onset.
Analysis and further testing
Aenean vitae neque est. Proin aliquam, lacus non ullamcorper elementum, diam eros faucibus orci, sed lobortis lectus urna egestas ligula. Morbi viverra, orci ac sodales consectetur, erat justo dictum urna, sodales ultrices nulla diam placerat ipsum. Donec at aliquet est. Aenean tincidunt vitae quam eu vestibulum. Cras ac velit ante. Maecenas aliquam purus nec tellus aliquet, id vehicula enim elementum.
Phasellus egestas elit eget porttitor sodales. Integer sollicitudin sollicitudin ornare. Morbi vel metus pellentesque, dignissim lacus vel, consequat metus. Nunc commodo viverra nunc, sed interdum nisl laoreet sed. Nulla facilisi. Vivamus tempus, justo id rhoncus egestas, lacus mauris vestibulum tellus, a pretium enim est sit amet mi. Etiam felis nisl, tincidunt et dictum id, condimentum ac lectus.
A positive result
Cras congue justo vitae tortor aliquam mollis vel eu orci. Nunc tincidunt dui interdum, dignissim odio sit amet, sodales tortor. Fusce dignissim faucibus est, sit amet mattis nunc sollicitudin eget. Ut eu viverra dolor. Mauris at velit purus. Duis non enim non libero mollis maximus nec id est.
Aenean vulputate felis vitae lacus consectetur, sit amet aliquet erat faucibus. Integer eget massa luctus, iaculis metus vitae, porta dolor. Morbi congue, velit vel fringilla tempor, leo mi viverra enim, vel dictum ipsum libero sed nisl. Pellentesque porta magna eros, a dignissim erat tempus ac. Nullam malesuada euismod laoreet. Fusce a lacinia erat.